UV Disinfection
Ushio has used its technical expertise to develop innovative light solutions for the fulfilment of ultraviolet (UV) disinfection and vacuum-ultraviolet (VUV) / ozone (O3) purification applications. Ushio’s lamps are renowned for their high efficiency and range of specialisation. This versatile technology is used in a vast array of disinfection applications, including surface cleaning, chemical-free ballast water treatment, and developed the PureRelease module which contains an instant-on UV device for water disinfection at the point of dispensation (POD). More recently, the Care222® line of products has signalled a new era of area disinfection after being found to be extremely effective at inactivating bacteria and viruses.
Please click the icons below to discover the Ushio solutions designed specifically for your application, or scroll down for more information about UV disinfection.
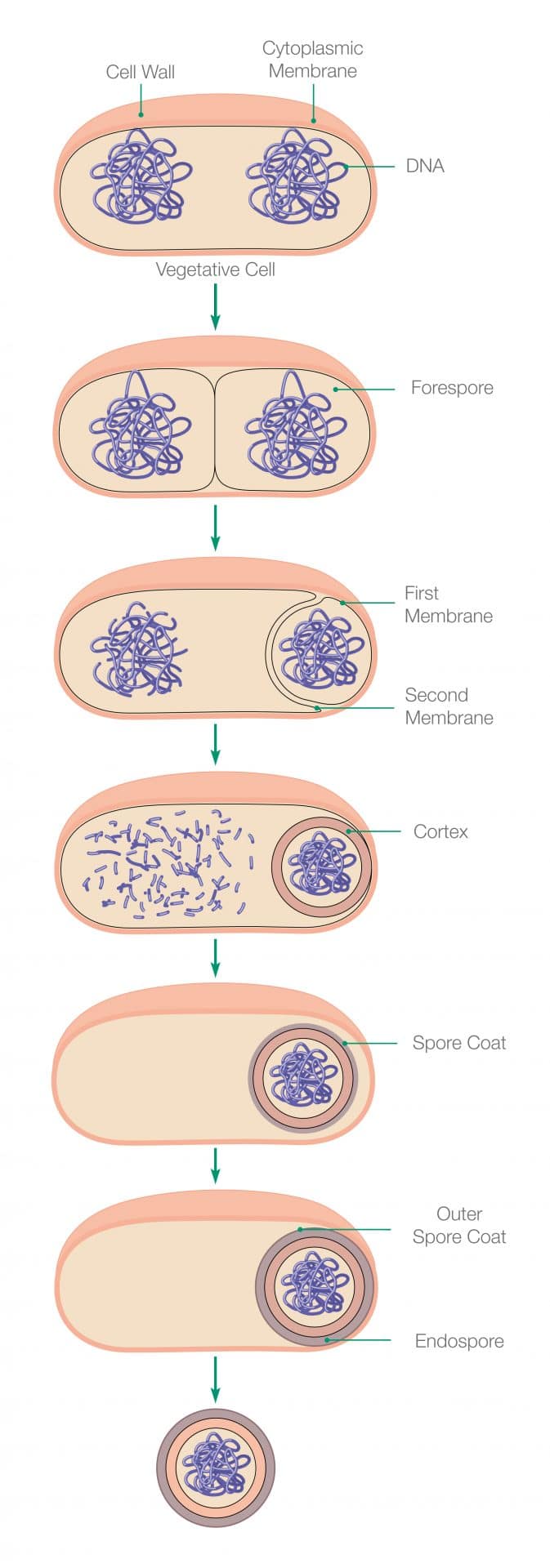
Endospores: Genetic Disinfection-Resistance
Disinfection and Sterilisation are both methods which achieve decontamination through the removal of pathogens. Many people incorrectly consider these terms to be interchangeable, but they have an important difference in meaning pertaining to the level of cleanliness achieved. The distinction between the two terms hinges on the post-treatment presence of endospores.
The proliferation of bacteria can be astonishing when they find themselves in favourable environmental conditions; however their genetic ability to survive is really something else. When the germ cell detects the presence of environmental stressors, it is able to metamorphosise into a protective state of hibernation until the environment returns to a suitable state. The cell is able to do this through a process called sporulation, which results in the formation of an endospore.
The bacterial cell achieves this by triggering a function within its genes to protect itself inside a newly toughened cell wall. Inside the cell wall, the developing endospore contains all the critical cellular elements required to ‘reactivate’ when better conditions arise. The original cell degrades away, leaving only the endospore shell behind. During this dormant phase, no signs of life can be detected within the endospore until a preferential environment returns. When that happens, the endospore germinates and exits the endospore coating to begin development into a fully functional vegetative bacterial cell again. The division process of the cell can then begin, leading to the resumption of bacterial propagation.
What is the Difference Between Disinfection and Sterilisation?
Disinfection is a process in which the number of pathogenic microorganisms, such as viruses and bacteria, is reduced through inactivation. Disinfection is ineffective against bacterial endospores despite being able to remove pathogens, which is what separates this type of cleaning from sterilisation.
Various methods of disinfection are commonly used throughout daily life, mostly for the acceptable decontamination of surfaces, water, and air. In a hospital scenario, low-level disinfection is implemented on noncritical surfaces and equipment which come into contact with intact skin, such as bed frames or the inflatable cuff of a blood pressure monitor. High-level disinfection must be implemented on semi-critical equipment which comes into direct contact with mucous membranes or non-intact skin; this includes devices such as endoscopes and respiratory therapy equipment.
Sterilisation is a process in which all pathogenic microorganisms, including vegetative endospores, are completely destroyed. Sterilisation kills, eradicates, and / or inactivates biological lifeforms such as bacteria, endospores, fungi, prions, and viruses.
Within healthcare practices, equipment sterilisation is a mandatory process which must take place before every use. Critical medical and surgical devices enter parts of the body tissue which are usually sterile or pertain to the flow of sterile bodily fluids such as blood and urine.
Ushio provided its VUV excimer technology as a component to SteriLux SA, who then designed and manufactured a medical instrument sterilisation device for use in developing countries. Most sterilisation processes involve autoclaves which use high temperatures (exceeding 100°C or 212°F) to kill endospores on medical instruments. However, these devices are usually very large and consume a large amount of electricity and water. This often means they are not a viable option in facilities suffering from vital supply issues. SteriLux SA successfully developed a device which reduces these factors while delivering an “A grade” solution to medical professionals around the world.
For further information regarding the SteriLux project, you can read our extensive success story here, which documents this groundbreaking partnership.
Ultraviolet Germicidal Irradiation (UVGI)
UV-C radiation (100 nm to 280 nm) is significantly involved in disinfection, water purification, and lithography solutions. Naturally occurring UV-C rays do not reach the surface of our planet due to the filtration effect of oxygen and ozone within the atmosphere of our planet.
Ushio’s years of research have allowed it to pinpoint UV wavelengths between 160 nm and 310 nm which have a unique germicidal ability to provide a means of disinfection through the inactivation of microorganisms and pathogens such as bacteria, viruses, and protozoa. Non-ionising radiation, such as ultraviolet light, has a mutagenic effect on living organisms by exciting the molecules at the very core of their DNA and RNA. The catastrophic destruction of the cell walls renders them unable to reproduce or infect and, in some cases, kills them.
Able to neutralise living cells in a matter of seconds, UVGI is an ideal alternative to chemical disinfection. The use of chemicals is detrimental to the environment, and in most cases, can lose effectiveness over time due to the ability of microorganisms to develop a resistance to them. There is no living cell with a total resistance to ultraviolet light, it occurs naturally within the environment, and does not affect the taste or smell of a UVGI treated substance.

UV / UVGI Disinfection Applications
- Air Purification
- Ballast Water Management
- Bottling & Packaging
- Conveyor Belt Systems
- Dairy Production
- Food & Beverages Production
- Germicide
- Industrial Kitchens
- Medical Science and Preparation
- Surface Treatment
- Volatile Organic Compound (VOC) Destruction
- Waste Water Treatment

Ushio secures exclusive patent from Colombia University; discover human-safe UV wavelengths with Kobe University
The Care222® product is presently used for unoccupied spaces with ongoing studies to make Care222® modules your future choice for all occupied and unoccupied microbial reduction solutions. Ushio’s longstanding collaborations with scientific institutions, such as Colombia University in the U.S.A., have contributed to the development of a system which selectively kills bacteria and viruses without damaging human cells/tissues using narrow-band spectrum UV. This has been made possible through the use of Colombia’s patented wavelength filtration technology. The system has demonstrated its ability to inactivate all kinds of ‘super bugs’ such as MRSA, influenza, MERS-CoV, as well as killing viruses including the Ebola virus.
Click here to read about our groundbreaking partnership with Kobe University, in Japan, which discovered that the 222 nm UV wavelength retains the disinfectant properties of 254 nm, yet does so without the hazardous health effects associated with prolonged exposure.
Visit our downloads page for the latest Ushio Europe product brochures.
Begin your product enquiries today by filling in the form on our contact page.