Excimer Technology: Understanding the science behind high-energy UV emission
The term excimer (a portmanteau of “excited” and “dimer” coined in the 1960s) refers to a temporary atomic state in which high-energy atoms form short-lived molecular pairs, or dimers, when electronically excited. These pairs are known as excited dimers. When these excited dimers return to their original state, the excess energy is released as ultraviolet (UV) light, specifically in the form of ultraviolet C (UVC) photons.
Contact
What Makes an Excimer Different?
By definition, an excimer refers to a dimer (a molecule made of two atoms) where both atoms come from the same element or species. For example, in a xenon (Xe) excimer lamp, high-energy xenon atoms (Xe) combine to form excited Xe2 dimers. When these dimers return to their ground state, they release UV photons with a wavelength of 172 nm. This wavelength is particularly useful in industry for surface activation, such as cleaning or modifying materials at a molecular level.
On the other hand, when molecules from two different elements form an excited complex, it's called an exciplex. A common example is the krypton-chloride (KrCl) exciplex, which emits UV light at a wavelength of 222 nm. This particular wavelength is highly valued for its ability to effectively kill harmful microorganisms, making it ideal for disinfection applications.
Excimers and Excimer lamps: What's the Connection?
While the term excimer technically refers to homonuclear dimers (pairs of the same element), it is often used more broadly to refer to both excimer and exciplex molecules, especially when discussing their use in technology. This broader usage has led to the term excimer lamp, which refers to devices that generate excimer (or exciplex) radiation, typically through discharge-based methods.
How do excimer lamps work?
Excimer lamps are specialized emitters filled with noble gases and coated to irradiate specific vacuum-ultraviolet (VUV) wavelengths, depending on the gases inside the sealed quartz glass chamber.
When high-energy electrons are introduced into the lamp, a plasma discharge is created, also known as a dielectric barrier discharge. This plasma contains high-energy electrons that excite the noble gas atoms inside the lamp, triggering the formation of excited dimers.
As these dimers return to their ground state in nanoseconds, they release UV radiation at specific wavelengths. The emitted photons travel through the air, where they are partially absorbed by oxygen to form ozone on the substrate. This ozone then breaks the molecular bonds of the surface.
The result is the removal of some substrate molecules, leaving behind a chemically modified top layer. Ushio’s excimer lamps, which can emit UV-C light at wavelengths from 172 nm to 310 nm, offer this effect along the entire length of the lamp. The specific wavelength is determined by the specific noble gas mixture used in the lamp.
222 nm UV-C and its disinfection benefits
The 222 nm wavelength, also known as "far UV-C", is gaining attention for its ability to disinfect surfaces without damaging organic tissue. This means that 222 nm lamps and modules, unlike 254 nm UV-C lamps, can be used in areas where people are present, when used within the current exposure limits defined by national regulations. Ushio’s Care222™ UV disinfection solutions are based on this wavelength.
Industrial applications and the 172 nm wavelength
In industrial settings, the most commonly used excimer wavelength is 172 nm. With an energy of 7.23 eV, it effectively breaks the molecular bonds of most organic molecules—except for those between carbon and oxygen (C=O) and some inorganic oxides. This makes 172 nm ideal for surface modification like cleaning, activation, and matting.
Applications of excimer irradiation: Enhancing surface performance
Excimer irradiation offers a range of benefits for various industries, improving surface properties and enabling new applications. Below are some key examples of how excimer technology is used:
1 Surface energy and wettability
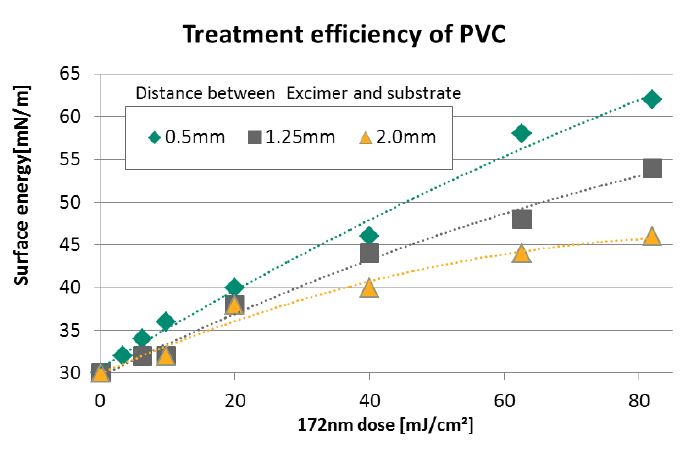
Excimer irradiation activates the surface of materials by increasing surface energy, which is measured in millinewtons per meter (mN/m). Surface energy determines the contact angle between a fluid and a solid surface. For effective adhesion and uniform distribution in applications like printing and gluing, it’s essential to achieve a low contact angle.
By increasing surface energy, excimer treatment enhances wettability, making it ideal for applications that require strong adhesion or even uniform coating layers.
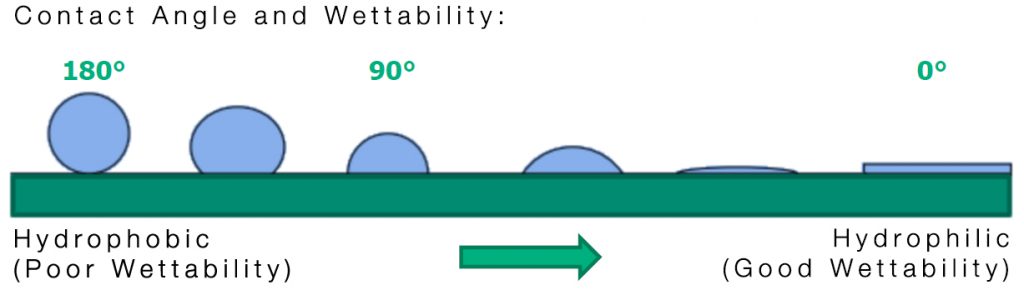
- Fluid surface energy > Substrate surface energy: Poor wettability and adhesion
- Fluid surface energy = Substrate surface energy: 90° contact angle, average wettability
- Fluid surface energy < Substrate surface energy: Good wettability and adhesion
Surface Activation Energy Requirements
For inks, coatings, or adhesives to bond effectively, the surface must reach a certain level of surface energy. Excimer treatment raises this energy by modifying the top molecular layer, ensuring optimal wetting and adhesion.
- Solvent inks: ≥ 34 mN/m
- UV inks: 36 – 42 mN/m
- Water-based colour systems: 38 – 50 mN/m
- Coatings: 36 – 42 mN/m
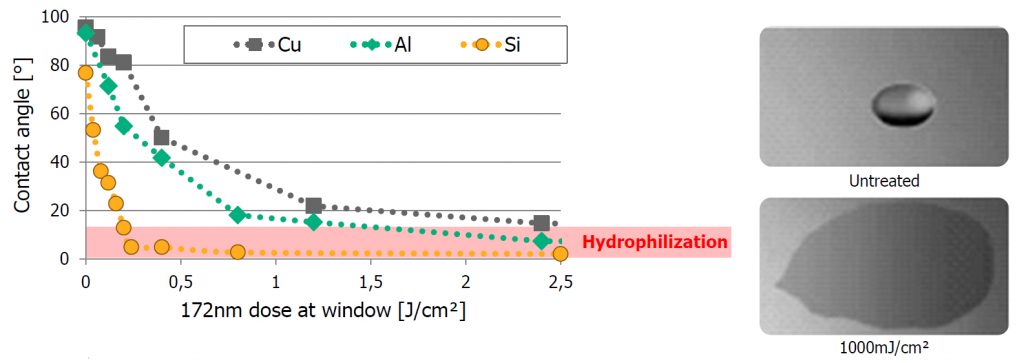
While the surface irradiation of polymers and composite materials is the most common application of excimer technology, the treatment can also be applied to almost any surface. Significant improvements in cleaning, coating, and bonding can be achieved on silicon wafers, metals, and glass.
In some cases, excimer treatment can induce complete hydrophilisation, meaning the liquid fully wets the surface. At this point, the contact angle reaches zero degrees, and the droplet transforms into a continuous liquid film — the ideal condition for uniform coatings and strong adhesion.
3 Adhesive-free bonding of materials
Ushio’s excimer lamps and modular solutions, backed by over 30 years of research and development, continue to push the boundaries of innovation. A recent breakthrough is the ability to directly print 3D electronics onto pre-treated surfaces, which could revolutionize industries where weight reduction is crucial, such as automotive and aviation.
Another exciting application is adhesive-free bonding. Excimer technology can bond certain materials directly, including polymers to quartz glass. The chart below shows examples of glass, mineral, and polymer materials that can be joined using excimer technology.
Ushio excimer irradiation solutions improve product quality
4 Improved printing quality
Excimer irradiation significantly enhances printing quality by modifying the surface chemistry of materials. In the comparison below, we see the difference between an untreated plastic card and a card treated with excimer UV light.

- Left (untreated card): Blurred barcodes and fuzzy text, uneven ink spread due to low surface energy.
- Right (treated card): Sharp, well-defined print with clean barcode edges. The excimer treatment has increased surface energy, improving adhesion for inks.
Key Benefits:
- Sharper lines and text
- No smudging or bleeding
- Durable, scratch-resistant print
Excimer treatment converts low-energy polymer surfaces into ink-friendly surfaces, ensuring high-contrast, long-lasting print quality on challenging materials such as PVC, PET, or polycarbonate.